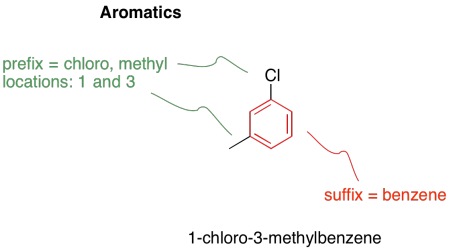
An aromatic is a particularly stable structure that is frequently seen in organic chemistry. Aromatics are planar, cyclic, fully conjugated systems with odd numbers of electron pairs in their conjugated π system. Benzene is the most common example of an aromatic molecule. It is a six-membered ring with alternating single and double bonds. It has three π bonds, and so it has three electron pairs in its π system.

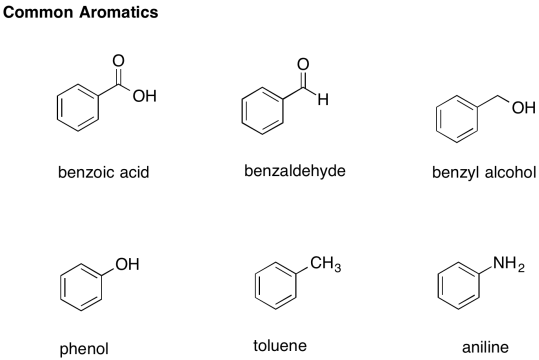
There are a number of common derivatives of benzene with names that you should remember.
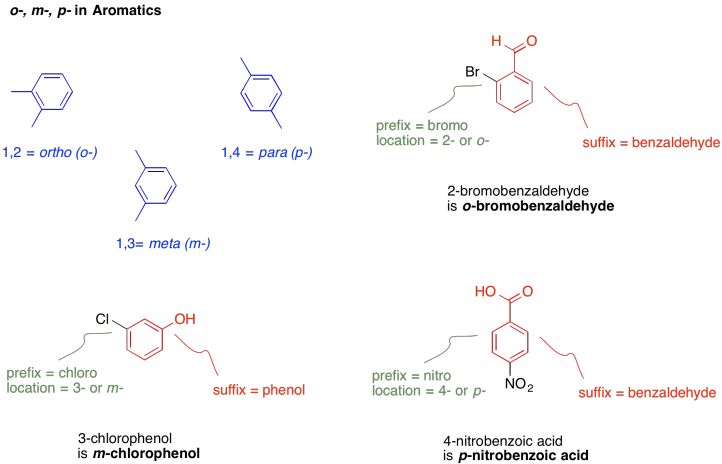
There is also a peculiar way of naming benzene derivatives that have two substituents on the benzene ring. The prefixes o-, m- and p- denote 1,2-disubstitution, 1,3-disubstitution and 1,4-disubstitution, respectively.
Problem FG61.
Draw the following compounds.
a) p-nitrotoluene b) o-chloroaniline c) m-methylphenol
d) m-chlorobenzyl alcohol e) o-bromobenzaldehyde f) p-fluorobenzoic acid
g) 2,4-dimethylphenol h) 2,5-dichlorotoluene i) 2,4,5-trimethylaniline
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by
Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send comments and corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.Navigation:
Back to Functional Group Appendix
Back to Web Materials on Structure & Reactivity in Chemistry